Beetroot (Beta vulgaris) is a root vegetable with a characteristic red colour that is due to the pigment betanin found within the vacuoles of beetroot cells.
Scientists investigated the effect of temperature on the release of betanin from the beetroot cells. This was recorded as the mean absorbance when using a colorimeter, with a higher absorbance indicating more pigment released. The scientists deduced that the pigment is released when the membrane proteins of the tonoplast surrounding the vacuole denature.
The graph below shows their results.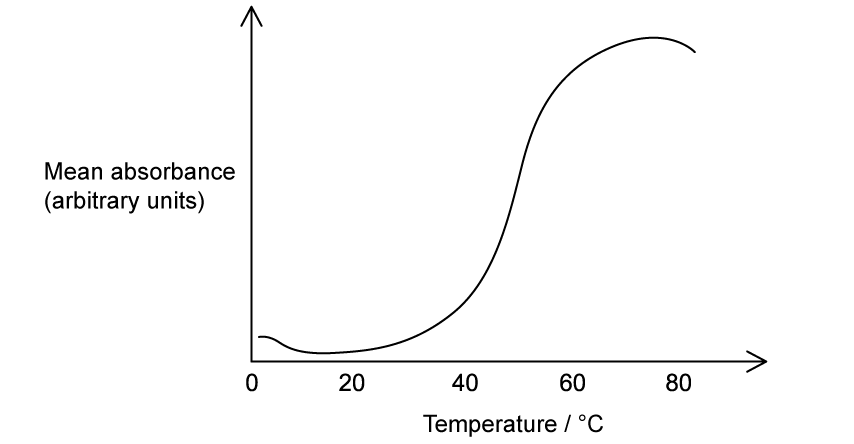
Which of the following descriptions would best explain the point where enzymes start to denature inside the beetroot cells?
Enzymes would denature at 30°C. At this point, the amount of pigment released from the beetroot cells is increasing and this indicates denaturing of the membrane proteins of the vacuole
Enzymes would denature when the temperature inside the beetroot cells increase to above 40°C. This is the point when membrane proteins begin to denature and release more pigment
The enzymes inside the beetroot cells would start to denature at 50°C. The greatest amount of pigment is released from the cells at this point which indicates that the membrane proteins of the vacuole are fully denatured
At temperatures above 60°C, the enzymes inside the beetroot cells will be denatured. Most of the pigment has been released at this point, indicating that the membrane proteins of the vacuole are denatured

















