Strong & Weak Acids
Strong acids
- A strong acid is an acid that dissociates almost completely in aqueous solutions
- Examples include HCl (hydrochloric acid), HNO3 (nitric acid) and H2SO4 (sulfuric acid)
- The position of the equilibrium is so far over to the right that you can represent the reaction as an irreversible reaction
Diagram to show the dissociation of a strong acid

The diagram shows the complete dissociation of a strong acid in aqueous solution
- The solution formed is highly acidic due to the high concentration of the H+/H3O+ ions
- Since the pH depends on the concentration of H+/H3O+ ions, the pH can be calculated if the concentration of the strong acid is known
- pH = -log10[H+ (aq)]
- [H+ (aq)] = concentration of H+ / H3O+ ions
- pH is the negative log of the concentration of H+ / H3O+ ions and can be calculated if the concentration of the strong acid is known using the stoichiometry of the reaction
Weak acids
- A weak acid is an acid that partially (or incompletely) dissociates in aqueous solutions
- E.g. most organic acids (ethanoic acid), HCN (hydrocyanic acid), H2S (hydrogen sulfide) and H2CO3 (carbonic acid)
- The position of the equilibrium is more towards the left and an equilibrium is established
Diagram to show the dissociation of a weak acid

The diagram shows the partial dissociation of a weak acid in aqueous solution
- The solution is less acidic due to the lower concentration of H+ / H3O+ ions
Acid & Equilibrium Position Table
| Strong Acids | Weak Acid | |
| Position of equilibrium | Right | Left |
| Dissociation | Completely (→) | Partially ( |
| H+ concentration | High | Low |
| pH | Use [strong acid] to calculate pH | Use Ka to find [H+] |
| Examples |
HCl HNO3 H2SO4 (first ionisation) |
Organic acids (ethanoic acid) HCN H2S H2CO3 |
- The strength of a Brønsted-Lowry acid depends on the ease with which it dissociates to release H+ ions
- This depends upon the strength of the bond that has to be broken to release H+
- For example, for hydrogen halides, the size of the halogen atom increases in size going down Group 17 which increases the length of the H–X bond
- As longer bonds are weaker they need less energy to break
- The acid strength of the hydrogen halides increases down Group 17
- HF < HCl < HBr < HI
Strong bases
- A strong base is a base that dissociates almost completely in aqueous solutions
-
E.g. group 1 metal hydroxides such as NaOH (sodium hydroxide)
- The position of the equilibrium is so far over to the right that you can represent the reaction as an irreversible reaction
-
Diagram to show the dissociation of a strong base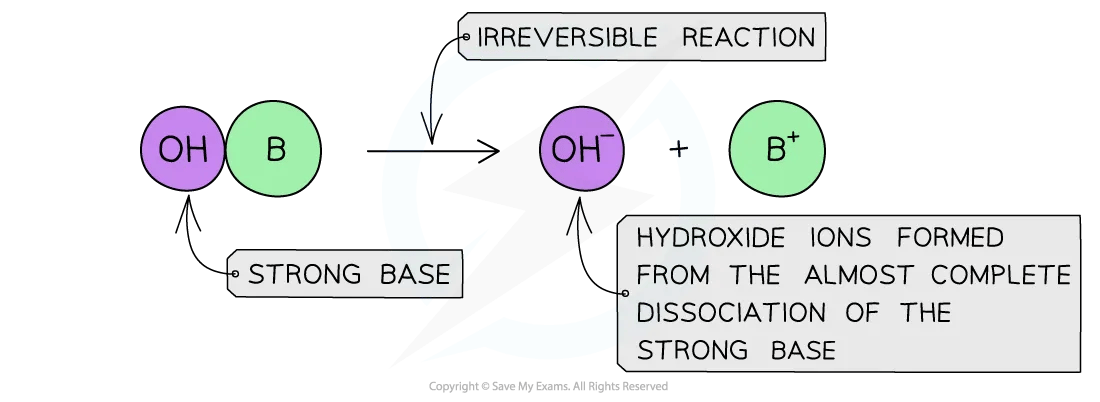
The diagram shows the complete dissociation of a strong base in aqueous solution
- The solution formed is highly basic due to the high concentration of the OH– ions
Weak bases
- A weak base is a base that partially (or incompletely) dissociates in aqueous solutions
- NH3 (ammonia), amines and some hydroxides of transition metals
- The position of the equilibrium is more to the left and an equilibrium is established
Diagram to show the dissociation of a weak base

The diagram shows the partial dissociation of a weak base in aqueous solution
- The solution is less basic due to the lower concentration of OH– ions
Base & Equilibrium Position Table
| Strong Base | Weak Base | |
| Position of equilibrium | Right | Left |
| Dissociation | Completely (→) | Partially ( |
| OH– concentration | High | Low |
| Examples |
Group 1 metal hydroxides |
NH3 Amines Some transition metal hydroxides |
Strength of conjugate acids and bases
- The conjugate base of HCl is the chloride ion, Cl–,
- However, since the reverse reaction is virtually non-existent the chloride ion must be a very weak conjugate base
HCl (g) → H+ (aq) + Cl– (aq)
acid conjugate base
- In general, strong acids produce weak conjugate bases and weak acids produce strong conjugate bases
- A strong base is also fully ionised and is a good proton acceptor
- For example, the hydroxide ion is a strong base and readily accepts protons:
OH– (aq) + H+ (aq) ⇌ H2O (l)
- The conjugate acid of the hydroxide ion is water, which is a weak conjugate acid
- In general strong bases produce weak conjugate acids
Exam Tip
- Hydrogen ions in aqueous solutions can be written as either as H3O+ or as H+
- However, if H3O+ is used, H2O should be included in the chemical equation:
HCl (g) → H+ (aq) + Cl- (aq) OR HCl (g) + H2O (l) → H3O+ (aq) + Cl- (aq)
- Some acids contain two replaceable protons (called 'dibasic')
- For example, H2SO4 (sulfuric acid) has two ionisations
- H2SO4 acts as a strong acid: H2SO4 → H+ + SO4-
- HSO4- acts as a weak acid: HSO4- ⇌ H+ + SO42-
- The second ionisation is only partial which is why the concentration of 1 mol dm-3 sulfuric acid is not 2 mol dm-3 in H+ ions
- For example, H2SO4 (sulfuric acid) has two ionisations
- Also, don't forget that the terms strong and weak acids and bases are related to the degree of dissociation and not the concentration
- The appropriate terms to use when describing concentration are dilute and concentrated
How to distinguish between strong and weak acid
- Strong and weak acids can be distinguished from each other by their:
- pH value (using a pH meter or universal indicator)
- Electrical conductivity
- Reactivity
pH value
- An acid dissociates into H+ in solution according to
HA → H+ + A-
pH value of a Strong Acid & Weak Acid Table
| Acid | pH of 0.1 mol dm-3 solution |
| HCl (strong) | 1 |
| CH3COOH (weak) | 2.9 |
- The stronger the acid, the greater the concentration of H+ and therefore the lower the pH
Electrical conductivity
- Since a stronger acid has a higher concentration of H+ it conducts electricity better
- Stronger acids therefore have a greater electrical conductivity
- The electrical conductivity can be determined by using a conductivity meter
- Like the pH meter, the conductivity meter is connected to an electrode
- The conductivity of the solution can be read off the meter
Diagram to show how to measure the electrical conductivity of an acid

A digital conductivity meter measures the electrical conductivity of a solution using an electrode
Reactivity
- Strong and weak acids of the same concentrations react differently with reactive metals
- This is because the concentration of H+ is greater in strong acids compared to weak acids
- The greater H+ concentration means that more H2 gas is produced in a shorter time
Diagram to show how a strong acid reacts with magnesium

The diagram shows the reaction of 0.1 mol dm-3 of a strong acid (HCl) with Mg. The reaction produces a lot of bubbles and hydrogen gas due to the high concentration of H+ present in solution
Diagram to show how a weak acid reacts with magnesium

The diagram shows the reaction of 0.1 mol dm-3 of a weak acid (CH3COOH) with Mg. The reaction produces fewer bubbles of hydrogen gas due to the lower concentration of H+ present in solution
- Similar observations would be made in the reaction between strong and weak acids with carbonates and hydrogencarbonates, although the gas given off this time is carbon dioxide
- With oxides and hydroxides, there may not be a lot of visible changes although it is likely that they would dissolve faster in a strong acid than in a weak acid
- These reactions are also likely to produce larger enthalpy changes which could be seen in higher temperature rises
Exam Tip
- The above-mentioned properties of strong and weak acids depend on their ability to dissociate and form H+ ions
- Stronger acids dissociate more
- This means that they produce a greater concentration of H+ ions resulting in:
- Lower pH values
- Greater electrical conductivity
- More vigorous reactions with reactive metals.
- This means that they produce a greater concentration of H+ ions resulting in:

