Percentage Error
- Percentage error is used to express the difference between a final calculated answer and an accepted or literature value
- It is calculated using the following formula
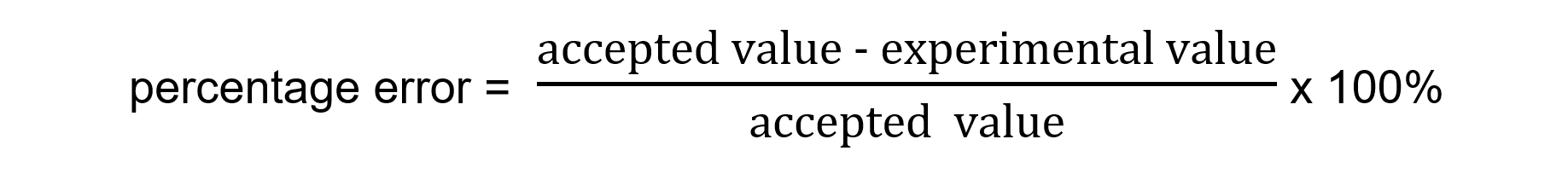
- You should be able to comment on any differences between the experimental and literature values
Worked example
1.023 g of propan-1-ol (M = 60.11 g mol-1) was burned in a spirit burner and used to heat 200 g of water in a copper calorimeter. The temperature of the water rose by 30 oC.
Answer 1:
Step 1: Calculate q
q = m x c x ΔT
q = 200 g x 4.18 J g-1 K-1 x 30 K = – 25 080 J
Step 2: Calculate the amount of propan-1-ol burned
moles = mass ÷ molar mass = 1.023 g ÷ 60.11 g mol-1 = 0.01702 mol
Step 3: Calculate ΔH
ΔH = q ÷ n = -25 080 J ÷ 0.01702 mol = – 1 473 560 J = -1 474 kJ = -1.5 x 103 kJ
Answer 2:
Using the formula
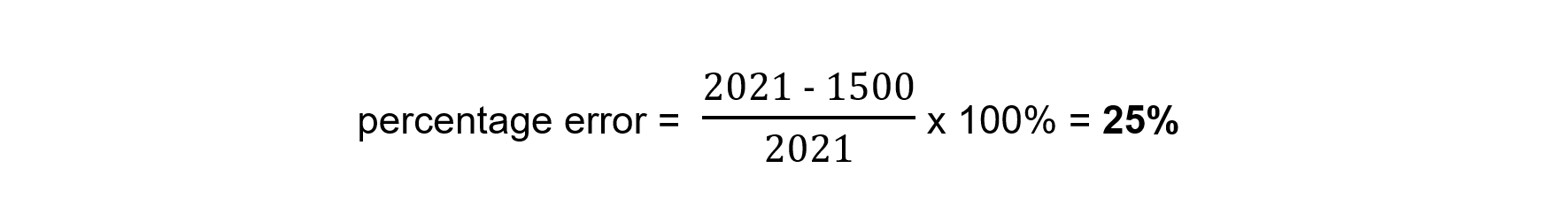
Heat losses are likely to be the largest source of error in this experiment
