Factors Affecting Colour
- The size of the splitting energy ΔE in the d-orbitals is influenced by the following four factors:
- The size and type of ligands
- The nuclear charge and identity of the metal ion
- The oxidation state of the metal
- The shape of the complex

The large variety of coloured compounds is a defining characteristic of transition metals
Size and type of ligand
- The nature of the ligand influences the strength of the interaction between ligand and central metal ion
- Ligands vary in their charge density
- The greater the charge density; the more strongly the ligand interacts with the metal ion causing greater splitting of the d-orbitals
- The further it is then shifted towards the region of the spectrum where it absorbs higher energy
- As a result, a different colour of light is absorbed by the complex solution and a different complementary colour is observed
- This means that complexes with the same transition elements ions, but different ligands, can have different colours
- For example, the [Cu(H2O)6]2+ complex has a light blue colour
- Whereas the [Cu(NH3)4 (H2O)2]2+ has a dark blue colour despite the copper(II) ion having an oxidation state of +2 in both complexes
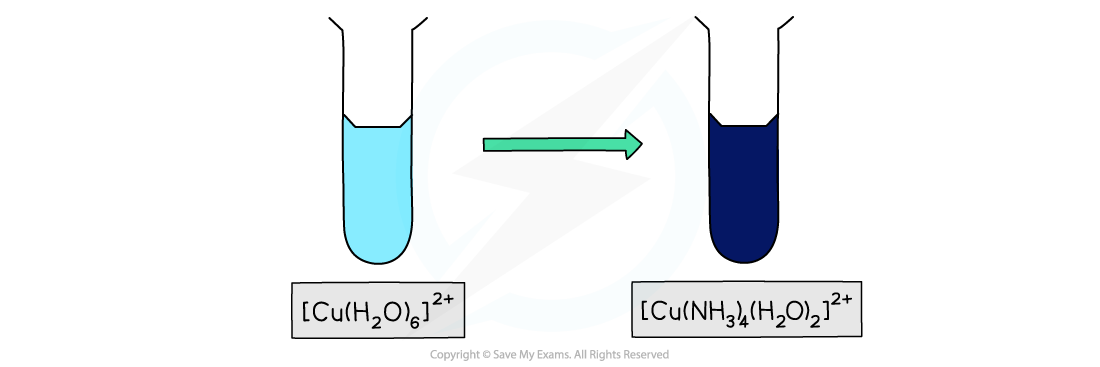
Ligand exchange of the water ligands by ammonia ligands causes a change in colour of the copper(II) complex solution
- Ammonia has a greater charge density than water and so produces a larger split in the d-orbitals

Graph showing the replacement of the water molecules with four ammonia molecules causes a shift in maximum absorbance towards shorter wavelength
The nuclear charge
- The strength of the attraction between the metal ion and lone pairs of electrons from the ligand can vary depending on the effective nuclear charge on the metal ion
- For example, aqueous Mn(II) and Fe(III) have the same electronic configuration:
[Ar] 3d5
- Mn(II) (Z=25) absorbs in the green region of the spectrum so appears pink
- The higher effective nuclear charge on Fe(III) (Z= 26) causes a stronger interaction with the ligands, so it absorbs in the higher energy blue part of the spectrum and appears yellow/orange in colour
Oxidation state
- When the same metal is in a higher oxidation state that will also create a stronger interaction with the ligands
- If you compare iron(II) and iron (III):
- [Fe(H2O)6]2+ absorbs in the red region and appears green
- But, [Fe(H2O)6]3+ absorbs in blue region and appears orange
Shape
- The change of colour in a complex is also partly due to the change in coordination number and geometry of the complex ion
- The splitting energy, ΔE, of the d-orbitals is affected by the relative orientation of the ligand as well as the d-orbitals
- Changing the coordination number generally involves changing the ligand as well, so it is a combination of these factors that alters the strength of the interactions


