Ultraviolet light is incident on a zinc plate. The zinc plate is situated within an evacuated chamber a few millimetres under a collecting plate, as shown in the diagram.
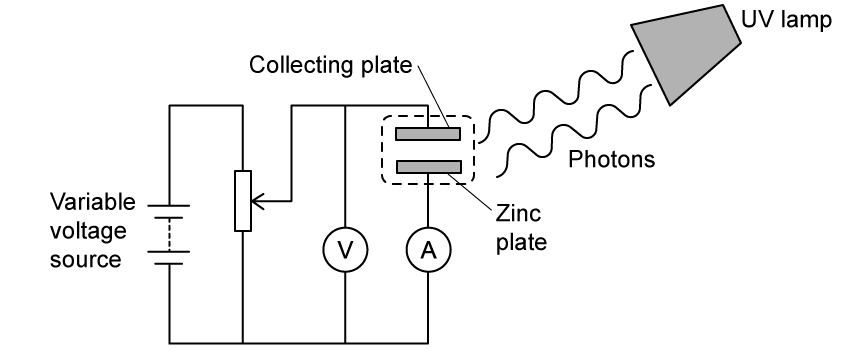
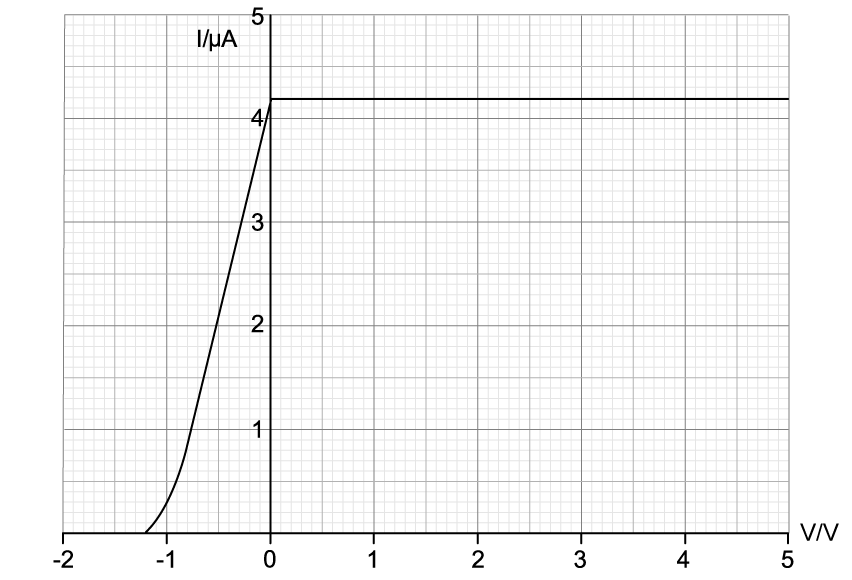
Photoelectrons are emitted from the zinc plate and move towards the positive collecting plate due to the potential difference, V, between the plates. When the potential difference, V, is varied, it is observed that the photoelectric current varies as shown on the graph.
(i)
Explain why the photoelectric current reaches a maximum value despite further increases in potential difference.
[2]
(ii)
The battery connections are reversed so that the potential difference across the plates is negative. As a result, the photoelectrons are now repelled by the collecting plate, although some still make it across.
Explain this observation.
[2]














