Alloys
What is an alloy?
- Alloys are mixtures of metals, where the metals are mixed together physically but are not chemically combined
- They can also be made from metals mixed with nonmetals such as carbon
- Ions of the different metals are spread throughout the lattice and are bound together by the delocalized electrons
- It is possible to form alloys because of the non-directional nature of the metallic bonds
Particle Diagram of an Alloy
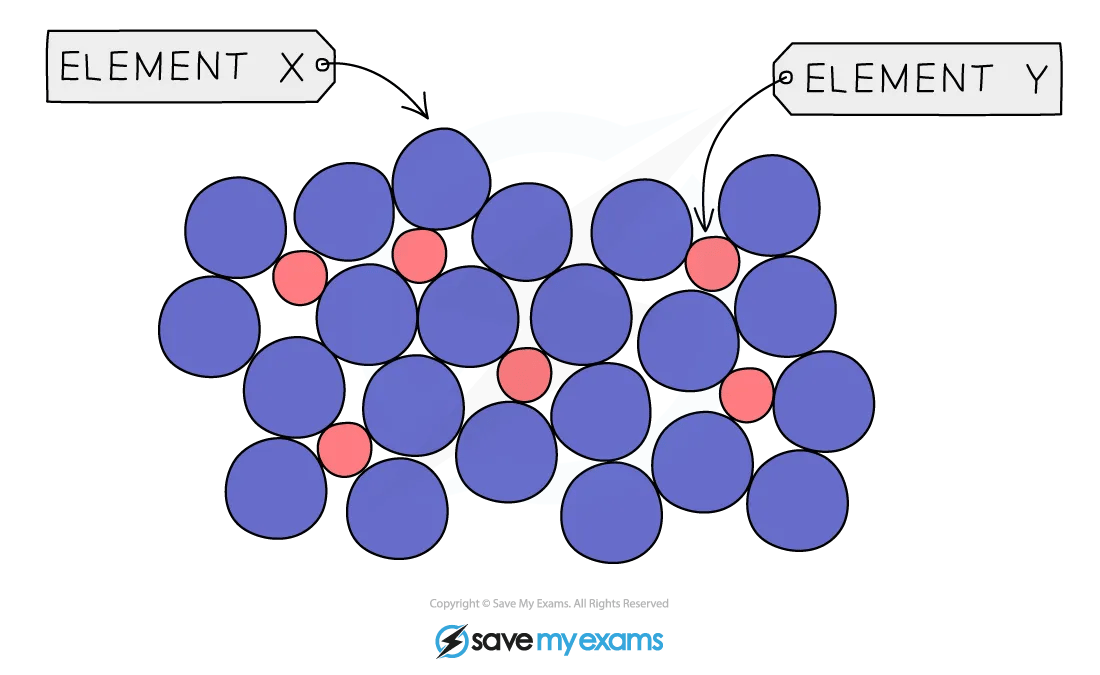
In a metallic lattice the regular structure of metal cations (shown by Element Y) is disrupted by the presence of another element (Element X)
Why do alloys have different properties to pure metals?
- Alloys have distinct properties due to the different packing of the cations in the lattice
- Alloys often have properties that can be very different to the metals they contain, for example they can have greater strength, hardness or resistance to corrosion or extreme temperatures
- Alloys contain atoms of different sizes, which distorts the regular arrangements of cations
- This makes it more difficult for the layers to slide over each other, so they are usually much harder than the pure metal
- Below is a table of some common alloys and their uses:
Common Alloys and their Uses Table
| Alloy | Elements present | Properties | Uses |
| Brass | copper and zinc | strong and resistant to corrosion | door handles, hinges, musical instruments |
| Steel | iron, carbon and other elements like chromium, vanadium and molybdenum | very strong | construction, bridges, cars |
| Stainless Steel | iron, chromium, nickel, carbon | corrosion resistant | cutlery, surgical instruments, cookware |
| Solder | lead and tin | low melting point | joining metals in electrical circuits and jewellery |
| Bronze | copper and tin | hard and strong resistance to corrosion | medals, sculptures, ship fittings |
Exam Tip
You don't need to learn the specific alloys, but you should be able to use examples you know to explain why alloys have the properties they do compared to pure metals

