Measuring Variables in Chemistry
- You need to know how to accurately measure variables to allow the collection of valid and high-quality data
- Sometimes, you will be required to make a decision as to what piece of equipment to use based on which is the most appropriate for that particular task
Measuring mass
- Mass is measured using a digital balance which normally gives readings to two decimal places
- Balances must be tared (set to zero) before use
- The standard unit of mass is kilograms (kg) but in chemistry, grams (g) are most often used
- 1 kilogram = 1000 grams
Measuring the volume of liquids
- The volume of a liquid can be determined using several types of apparatus, depending on the level of accuracy needed
- For approximate volumes where high accuracy is not an important factor, measuring (or graduated) cylinders are used
- These are graduated (have a scale so can be used to measure) and are available typically in a range of sizes from 10 cm3 to 1 litre (1 dm3)
- Volumetric pipettes are the most accurate way of measuring a fixed volume of liquid, usually 10 cm3 or 25 cm3
- They have a scratch mark on the neck which is matched to the bottom of the meniscus to make the measurement
- Burettes are the most accurate way of measuring a variable volume of liquid between 0 cm3 and 50 cm3 (e.g. in a titration)
- The tricky thing with burettes is to remember to read the scale from top to bottom as 0.00 cm3 is at the top of the column
- Whichever apparatus you use, you may see markings in ml (millilitre) which is the same as a cm3
Equipment used to measure the volume of liquids
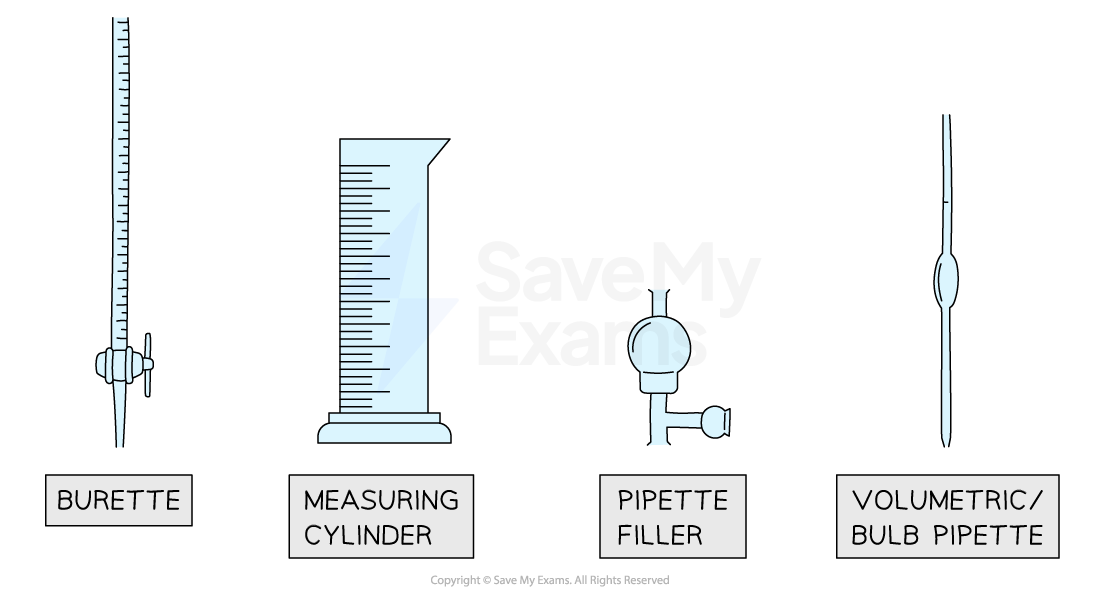
Diagram of a burette, a measuring cylinder, a pipette filler and a volumetric pipette
Measuring the volume of gases
- The volume of a gas sometimes needs to be measured and is done by collecting it in a graduated measuring apparatus
- A gas syringe is usually the apparatus used
- A graduated measuring cylinder or burette inverted in water may also be used, provided the gas is not water-soluble
- If the gas happens to be heavier than air and is coloured, the cylinder can be used upright
Measurement of the volume of gas using a gas syringe

Diagram of the set-up for an experiment involving gas collection
Measuring time
- Time can be measured using a stopwatch or stop-clock which are usually accurate to one or two decimal places
- The units of time normally used are seconds or minutes although other units may be used for extremely slow reactions (e.g. rusting)
- 1 minute = 60 seconds
- An important factor when measuring time intervals is human reaction time
- This can have a significant impact on measurements when the measurements involved are very short (less than a second)
Exam Tip
- Be careful when recording time not to mix up seconds and minutes in the same table
- If a table heading shows Time / mins and you record a stopwatch display of 1.30, meaning 1 minute and 30 seconds, that is wrong as it should be 1.5 mins
- To avoid any confusion, if the time intervals are less than a minute, it is best to change the recorded units to seconds
- So the 1.30 stopwatch display would therefore be recorded as 90 seconds
Measuring temperature
- Temperature is measured with a thermometer or digital probe
- Laboratory thermometers usually have a precision of a half or one degree
- Digital temperature probes are available which are more precise than traditional thermometers and can often read to 0.1 oC
- Traditional thermometers rely upon the uniform expansion and contraction of a liquid substance with temperature; digital temperature probes can be just as, if not, more accurate than traditional thermometers
- The units of temperature are degrees Celsius (ºC)
Measuring length
- Rulers can be used to measure small distances of a few centimetres (cm).
- They are able to measure to the nearest millimetre (mm)
- The standard unit of length is metres (m)
- Larger distances can be measured using a tape measure
- Many distances in chemistry are on a much smaller scale, for example, a typical atomic radius is around 1 x 10-10 m, so cannot be measured in this way
Measuring length

A ruler can measure distances to the nearest mm
Measuring the pH of a solution
- pH can be measured using an indicator or a digital pH meter
- pH meters contain a special electrode with a thin glass membrane that allows hydrogen ions to pass through; the ions alter the voltage detected by the electrode
- An indicator is a substance which changes colour depending on the pH of the solution to which it is added
- There are natural indicators and synthetic indicators which have different uses
- Generally, natural indicators are wide range indicators that contain a mixture of different plant extracts and so can operate over a broad range of pH values
- Synthetic indicators mostly have very narrow pH ranges at which they operate
- They have sharp colour changes meaning they change colour quickly and abruptly as soon as a pH specific to that indicator is reached
- Indicators are intensely coloured and very sensitive so only a few drops are needed
- Universal indicator is a wide range indicator and can give only an approximate value for pH
- It is made of a mixture of different plant indicators which operate across a broad pH range and is useful for estimating the pH of an unknown solution
- A few drops are added to the solution and the colour is matched with a colour chart which indicates the pH which matches with specific colours
- Universal indicator colours vary slightly between manufacturer so colour charts are usually provided for a specific indicator formulation
Colours of universal indicator

pH scale with the universal indicator colours used to determine the pH of a solution
Exam Tip
- pH probes offer higher precision and accuracy compared with indicators, so they are more suitable for most applications
- Indicators with a sharp colour change are still a suitable choice for use in titrations as they give a clear endpoint, are simple to use and give valid results
- pH meters may respond more gradually to changes in pH so may not provide a clear, sharp signal at the endpoint
Measuring electric current
- Current is measured using an ammeter
- Ammeters should always be connected in series with the part of the circuit you wish to measure the current through

An ammeter can be used to measure the current around a circuit
Digital or Analogue?
- Ammeters can be either
- Digital (with an electronic display)
- Analogue (with a needle and scale)
Analogue Ammeters
- Typical ranges are 0.1 - 1.0 A and 1.0 - 5.0 A for analogue ammeters
- Always double-check exactly where the marker is before an experiment
- If the marker is not at zero, you will need to subtract this from all your measurements
- They should be checked for zero errors before using
- They are also subject to parallax error
- Always read the meter from a position directly perpendicular to the scale
An analogue ammeter

Analogue ammeters have a needle and scale for measuring electric current
Digital Ammeters
- Digital ammeters can measure very small currents, in mA or µA
- Digital displays show the measured values as digits and are more accurate than analogue displays
- They’re easy to use because they give a specific value and are capable of displaying more precise values
- However, digital displays may 'flicker' back and forth between values and a judgement must be made as to which to write down
- Make sure the reading is zero before starting an experiment, or subtract the “zero” value from the end results
- Digital ammeters should be checked for zero errors
A digital ammeter

Digital ammeters have an electric read-out for measuring electric current
Measuring the electric potential difference
- Electric potential difference is measured using a voltmeter, which can be either
- Digital (with an electronic display)
- Analogue (with a needle and scale)
- Voltmeters are connected in parallel with the component being tested
- The potential difference is the difference in electrical potential between two points, therefore the voltmeter has to be connected to two points in the circuit
Analogue or Digital?
- Analogue voltmeters are subject to
- Always read the meter from a position directly perpendicular to the scale parallax errors
- Typical ranges are 0.1-1.0 V and 0-5.0 V for analogue voltmeters although they can vary
- Always double-check exactly where the marker is before an experiment, if not at zero, you will need to subtract this from all your measurements
- They should be checked for zero errors before using
An analogue and digital voltmeter

Voltmeters can be either analogue (with a scale and needle) or digital (with an electronic read-out) for measuring the electric potential difference
- Digital voltmeters can measure very small potential differences, in mV or µV
- Digital displays show the measured values as digits and are more accurate than analogue displays
- They’re easy to use because they give a specific value and are capable of displaying more precise values
- However, digital displays may 'flicker' back and forth between values and a judgement must be made as to which to write down
- Digital voltmeters should be checked for zero errors
- Make sure the reading is zero before starting an experiment, or subtract the “zero” value from the end results

